iHealthScreen Seeks FDA Approval for AI-Powered AMD Tool
iHealthScreen, in a groundbreaking move, has successfully submitted for FDA 510(k) clearance for iPredict, positioning itself as the first-ever company to seek FDA approval for screening Age-Related Macular Degeneration (AMD), as stated in an official news release.
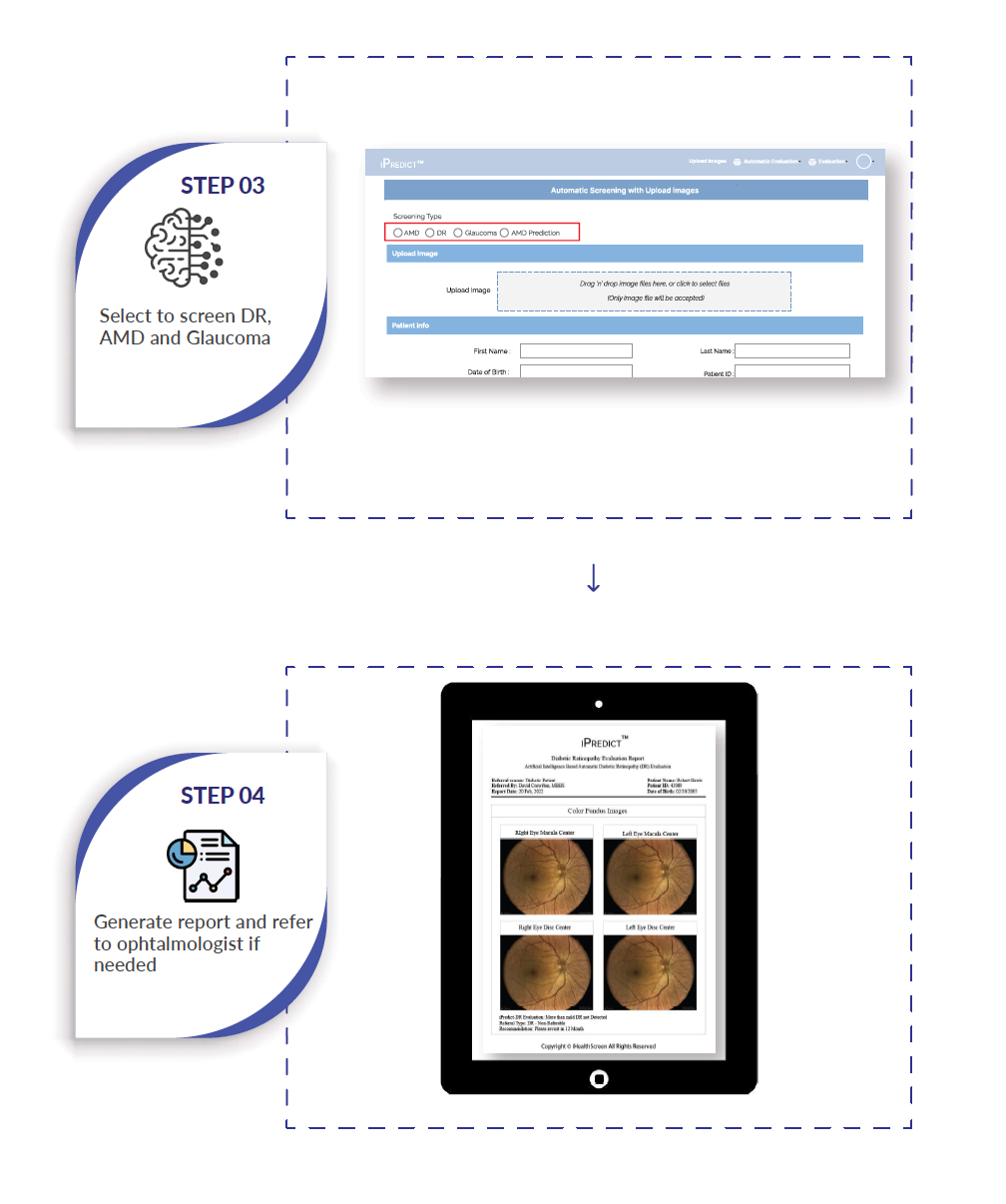
The innovative iPredict AI Eye Screening System offers a comprehensive solution for AMD screening, incorporating retinal imaging and providing immediate, actionable results. By leveraging the iPredict System, primary care physicians and various specialty practices gain the capability to accurately and efficiently screen individuals aged 50 and above for AMD, streamlining the referral process to ophthalmologists, as highlighted by iHealthScreen.
By utilizing a color fundus camera to capture high-resolution images of the patient's eyes, iPredict AI System delivers fully automated screening results in less than 60 seconds. Remarkably, the entire process can be completed in just 5 minutes, ensuring efficiency and reliability.
To validate the product's performance, iHealthScreen conducted a prospective trial involving a diverse sample from the general population. The pivotal trial showcased outstanding accuracy for iPredict-AMD, boasting a sensitivity of 86.86% and a specificity of 94.13%, as confirmed by iHealthScreen.
In its 510(k) application to the FDA, iHealthScreen has proposed the following indication for iPredict’s use:
- iPredict-AMD is indicated for use by health care providers to automatically detect more than early age-related macular degeneration (mteAMD) in adults above 50 years of age who have not been previously diagnosed with Late AMD (i.e., not legally blind or not showing blind spots in their visual field).
- iPredict-AMD is indicated for use with the DRSPlus color fundus camera (iCare Inc. K192113) in both primary care and eye care settings.
The results of this prospective study were originally unveiled at the prestigious annual meeting of the American Academy of Ophthalmology (AAO). The groundbreaking findings were acknowledged as outstanding work and were selected for a panel discussion, highlighting their significance and contribution to the field.
In cases where referable stage disease is detected for any of the conditions, the iPredict automated report advises an immediate visit to an ophthalmologist for suitable treatment. Conversely, adhering to established care standards, a follow-up visit after 1 year is recommended.
Designed for healthcare providers in clinics, hospitals, and various healthcare facilities, iPredict is specifically indicated for the automatic detection of AMD. Its purpose is to empower healthcare professionals in efficiently identifying and addressing this condition.
“This technology could be particularly useful in identifying someone who has slipped across the boundary for progression into severe AMD,” Theodore Smith, MD, Professor in Ophthalmology and Neuroscience at Icahn School of Medicine at Mount Sinai, New York, said in a company news release.